HYGIENE IN MILK PRODUCTION AND PROCESSING

Presentation – Hygiene
Downloads
Introduction and definitions
In the past, the ancient Greeks were familiar 4,000 years ago with the concept of hygiene. Asclepios (Asclepius), the god of medical skills named his daughter Higieia (Higia), and she was the goddess of health in ancient Greece. In Greek, her name meant “heal,” or to “bring health.” The ancient Greeks considered her a protector or personification of health, and her name has been preserved to this day in the word “hygiene.” Hygiene means a number of measures and procedures related to cleanliness, order and neatness that ensures health, so hygiene has become part of preventive medicine. Health can be defined as physical, spiritual and social well-being. Hygiene is a large part of our daily lives, from personal hygiene, housing hygiene, environmental hygiene, and nutrition hygiene. In this context, the hygiene in domestic animals in our breeding for the production of milk and meat deserves importance and should be promoted. Hygiene in milk production and processing has a decisive significance due to the increasing demands for quality, durability, health safety and safety of dairy products. Market requirements for these dairy properties are increasing and therefore the concept of “quality assurance” is introduced which requires mastery of the production process and full control over it from the beginning to the expiry of the product on the market. Classic product control according to ISO standards in its own laboratory and in accredited laboratories remains a tool that supports the concept of quality assurance. The fight for the health-correct dairy product begins in the pasture, the ploughland and the barn. This is the primary part of production which is also an important integral part of the HACCP system.
Definitions:
Cleaning/washing – removing dirt, leftover food, grease and other impurities.
Contaminant – any biological or chemical agent, foreign substance or substance that has not been intentionally added to food, which could jeopardize the health and suitability of food for consumption.
Contamination – introduction or appearance of contaminants in the food or environment in which the food is located.
Danger – biological, chemical or physical agent in food, or a food condition, which could have a negative effect on health.
Disinfection – reduction of the number of microorganisms in the environment with chemical agents and/or physical methods at the level that does not compromise the health safety and suitability of food for consumption.
Food – a food product (the term that covers the entire food chain – from raw materials, until possibly adding ingredients to the finalized consumable product).
Food handler – any person who directly handles packaged or unpackaged food, equipment and accessories used in food manufacturing or if it comes into contact with surfaces that contact food, and therefore from whom is expected to meet the requirements of food hygiene.
Food hygiene – all conditions and measures necessary to ensure the health safety and suitability of food for consumption.
HACCP – system that identifies, evaluates and controls hazards that are significant to the health correctness of food.
Health safety of food – assurance that food will not adversely affect the consumer, if prepared and/or consumed in the way intended for such food.
Industrial facilities – any complex or space where food is handled and the environment that is under the control of the same management.
Primary production – the faze in the food chain up to (and including) harvest, slaughter, milking, catch (fish) etc.
Risk – indicates the likelihood that the present potential danger occurs as an injury or illness in the work process. Danger is a qualitative term, and the risk is a quantitatively expressed as possibility that the existing danger actually leads to health damage.
Suitability of food – assurance that food is acceptable for human consumption in the way intended for such food.
Hazards that can be transmitted by contaminated milk
Danger is all that can harm the consumer of dairy products. The dangers can be of a physical nature, chemical substances, radioactive substances and foreign microorganisms as a microbiological hazard.
Physical hazards
Physical hazards that can get into milk are metal parts, sand, earth, animal litter, pebbles, wood, plastic, hair, rubber items, glass and personal jewelry. Such contaminants can be sources of microbiological hazards as well. The causes of this kind of contamination are poorly educated people, bad maintenance of equipment and process management.
Chemical hazards
Chemical hazards in milk are veterinary drugs, pesticides, insecticides, mycotoxins, cleaning and disinfectant agents. Antibiotics in milk are most often a consequence of mastitis treatment, as a very dangerous and expensive disease of dairy cows. Antibiotics negatively affect human health, but by their action they literally prevent the production of fermented dairy products from contaminated milk. Antihelminitic drugs are used to get rid of internal parasites. In the body of animals they are broken down and toxic metabolites are formed and therefore it is very important to observe the withdrawal period for antibiotics and for drugs against internal parasites. Pesticides are toxic substances intended to combat pests in agriculture. They can get into the organism of dairy cows by consuming feed and water, and then into milk. Disinfectant, disinsection and deratization agents are very dangerous and can only be administered by educated people and registered institutions, in cooperation with dairy experts. Cleaning and disinfectant agents may get into milk due to incomplete rinsing of dairy equipment. Also, due to improper selection and use, they can cause corrosion of dairy equipment, therefore heavy metals can get into milk. An example of this is the use of sodium hypochlorite that destroys dairy equipment made of some standard types of stainless steel.
Mycotoxins – some types of mold produce toxic metabolites that are dangerous to human health and are called mycotoxins. The most famous mold is Aspergillus flavus, and the toxin is aflatoxin. A known source of aflatoxin is a feed mixture contaminated with mycotoxins.

Figure 1. Morphological representation of mold Aspergillus flavus
Radioactive substances
A thermonuclear reactor explosion at the Chernobyl power plant on April 26, 1986 ejected 400 times more radionuclides into the atmosphere than a nuclear bomb dropped on Hiroshima in 1945. Radionuclides are radioactive elements that are formed in the process of uranium decay in a nuclear reactor, releasing a large amount of thermal energy. For human health, radionuclides of iodine, cesium and strontium are the most dangerous because they can enter the body with food and stay in their “physiological points” and thus irradiate the body from the inside. Milk can be brought into the body by radionuclides because cows can pick up large amounts of radionuclides from plants to which radionuclides have fallen with rain. Air currents and winds spread radionuclides all over the Europe and some of these radioactive substances fell on Croatian territory with rain and at the beginning of May of that year. The cows were grazing at the time, so radionuclides got into milk, and with dairy products got into the human body. Nuclear incidents are not excluded from the future, so the government should have plans to deal with such a crisis.
Microbiological hazards
Microorganisms in general, and bacteria as part of that world, play a very significant positive role in dairy industry, but also in a negative sense, as seen from our position.
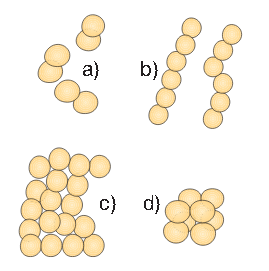
Figure 2. Spherical bacteria (cocci) occur in different formations
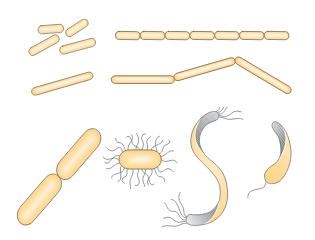
Figure 3. Bacteria in the form of sticks and spirals
Bacteria are single-celled microorganisms that multiply, reproduce by dividing the parent cell into two mutually equal daughter-cells, after a while which are divided in the same way. This method of multiplication is called binary fission or division into two parts. Their size is on average from 0.5 to 5 micrometers. Bacteria can be seen and studied under a microscope with a magnification of a thousand-fold, Figure 4.

Figure 4. Morphological appearance of bacterial cells
According to the coloring, bacteria are divided into Gram positive (blue) and Gram negative (red), and this is due to a significant difference in the cell structure, i.e. the chemical composition of the cell wall. The difference in the composition of the cell wall is reflected with difference in resistance to antibiotics, which means that some antibiotics act on Gram positive but not on Gram negative bacteria. Because of this, coloring by Gram has great significance in microbiology. Many bacteria can actively move in a liquid medium. The genera Bacillus and Clostridium can create highly resistant spores under harsh conditions that preserve and protect them until the conditions for active living are created. Spores can be destroyed in the autoclave using water vapor heated to 120°C during time of 20 to 30 minutes. If pipes are not washed regularly, some bacteria create mucosal capsules that protect them from dehydrating and can interlink them into compact layers on industrial pipeline walls. The growth of such bacteria in milk makes milk viscous, mucous and stringy. As other living beings, bacteria need nutrients for their growth and their basic sources of food are organic compounds; proteins, fats and carbohydrates. The mentioned substances serve bacteria to build their cells and produce energy. They also need trace elements and vitamins, and these substances must be soluble in water and have a small molecular mass in order to pass through the cytoplasmic membrane and enter the cell of the bacterium, and hence bacteria need water for their growth. Milk is an ideal medium for the growth of various types of bacteria and other microorganisms because it contains 87.5% water and all the necessary nutrients in abundance. Microorganisms can live in symbiosis, which means they help each other. Conversely, some microorganisms produce substances that inhibit the growth of other microorganisms, and this is called antibiosis.
Shapes of bacteria
- Spherical-shaped (cocci – coccos = core) – reminiscent of small balls. They can be single and these are monococcus. After the cellular division, they can stay together and form diplococcus. Streptococcus – from Greek “streptos” = chain – occurs after the division when spherical bacteria stay together and form shorter or longer chains. Staphylococcus – from Greek “staphyle” = cluster – occurs after the division when spherical bacteria stay together and form a cluster. Tetracoccus – from Greek “tetra” = four, when two pairs of globed bacteria stay together after the division. Sarcina is a form that occurs after division when eight globular cells remain together in the form of “packets.”
- Rod-shaped (bacillus-bacilli) – can be short or long, thin or thick, i.e. different lengths and diameters, with dull or sharp endings. Most often they appear individually. If the sticks do not separate after division but remain in pairs they are called diplobacillus. Streptobacillus occur when stick cells form longer or shorter chains after division. Palisades (from Greek “palus” = stake) are formed when the sticks are arranged side by side and resemble a “stake fence” after division.
- Curved-rod shapes differ according to the type of curving:
- vibrions – short shapes in the form of commas
- spirili – longer shapes with two or more curves, most often in the form of the letter “S”
- spirohete – shapes with larger number of sharper curves
- Stringy shapes represent a special form of bacteria. These include, for example, actinomycetes (hypha-forming bacteria). They are located at the transition between bacteria and mold. They have a branched web shape. Sulfur and iron bacteria have stringy shapes.
- OTHER shapes: e.g. astra (stella) – star shape; arcula – square shape
Water activity (aw)
The growth and metabolism of microorganisms depends on the presence of water in the accessible form because water can also exist in a bound inaccessible form. The measure for water availability is water activity (aw). Reducing the water content of food is a way to make food inaccessible to spoilage agents. For this reason, drying, salting and sugaring are/were mainly used procedures for preserving food. It should be emphasized that no microorganism can grow in food if the water activity is less than 0.6. It should also be known that depending on the condition before drying, dried food may contain live microorganisms including pathogenic bacteria and toxins. This in our case applies to milk powder. Only top-quality raw material and a well-managed processing can provide a quality product.

Figure 5. Impact of aw on the growth of microorganisms
Temperature
The strongest single factor of growth and multiplication of bacteria, and therefore spoilage of food. Bacteria can reproduce within certain temperature limits that vary from species to species. In principle, bacteria can grow at temperatures between the freezing point of the water and the temperature at which proteins in the cytoplasm are denatured. Optimal growth temperatures lie between maximum and minimum temperatures, i.e. upper and lower limitations. This is the temperature at which a particular type of bacteria multiplies fastest. Temperatures below the minimum stop growth, but do not kill bacteria. The life functions of bacteria almost completely cease at temperature near the freezing point of water. Bacterial cells contain from 75 to 98 % water, so when the temperature drops below the freezing, the water in the bacterial cell solidifies, so the bacteria can no longer absorb nutrients from the environment through the cell membrane, and it enters a state of survival. The freezing point of the milk is – 0.517°C, which means that the milk at that temperature is still liquid. If temperatures rise above maximum heat will kill bacteria quickly. Most cells die within seconds of being exposed to a temperature of 70°C. Some bacteria survive heating of up to 80 °C for 5 minutes, although they do not form spores. Destroying bacterial spores takes a lot more heat. Treatment with water vapor at 120°C for 30 minutes ensures the destruction of all spores. This effect is also achieved by dry heat, but at a temperature of 160°C for two hours.


Figure 6. and 7. Temperature conditions for bacterial growth
Bacteria can be divided into four categories according to the range of temperatures they prefer. Psycherophilic (prefer cold) bacteria grow well at 0 °C, their optimum temperatures are around 12-15 °C and maximum below 20 °C. Psyhrotrophic (tolerate cold) bacteria are mesophilic strains that can multiply at temperatures of commercial refrigerators, their optimum temperatures are about 20-30 °C. Mesophilic bacteria have minimum temperatures around 10 °C and generally optimum at 30-35 °C and maximum at about 50 °C. It is the most common temperature profile of bacterial growth. At this temperature interval, about 90% of all bacteria can grow. Thermophilic (prefer hot) bacteria have growth optimum at temperatures of 55-65 °C. The minimum temperature is about 37 °C and the maximum is about 70°C. Psyhrotrophic bacteria are of particular interest to dairy farming because the microbiological activity of milk on farms and in stores takes place at temperatures of 7 °C and lower! Therefore, freshly milked milk should be cooled at temperature of 2 °C.
Oxygen
The relationship between bacteria and oxygen is very complex because bacteria have taken incredible ecological niches on Earth. There are four groups: aerobes, microaerophiles, anaerobes and facultative anaerobes.
Light
Direct sunlight kills bacteria. Ultraviolet light in the solar rays causes changes in the DNA and proteins of bacterial cells.
Acidity (pH value)
Yeasts and mold grow best in a slightly acidic medium around pH 5-6. For bacteria, optimal conditions are neutral or poorly alkaline environment. Fresh milk has a pH value between 6.5 and 6.7 so it is a good nutrient for bacteria. Regarding acidity, liquid food is divided into strongly acidic foods and low-acidic foods. The boundary between those two zones is a pH value of 4.6. This has a major impact on milk processing and manufacturing of dairy fermented products. Pathogenic bacteria cannot grow below pH 4.6.

Figure 8. Impact of pH on microbiological growth
Bacterial growth
Bacteria multiply by binary division. Each individual cell grows and when it reaches a critical size it divides into two identical cells. The way, the type in which cells are grouped during division is constant for particular type of bacteria. It can be a chain, one pair of cells, a cube, a cluster or a “bunch”. This defines the appearance of bacterial colonies on nutritious substrates. Under favorable conditions, bacteria can be divided at intervals of 20 – 30 minutes, and we call this period generation time. The rate of bacterial reproduction is calculated by the formula:
 N- number of bacteria/ml after time t
N- number of bacteria/ml after time t
N0– number of bacteria/ml after time 0
t- growth time in hours
g- generation time in hours
With 0.5 hours of generation time, in ten hours one bacteria/ml can create about 10 million cells/ml. In enclosed system, bacterial growth will slow down and convert to stationary phase followed by the death of that type of bacteria. Reasons for impeding bacterial growth are the lack of nutrients and the accumulation of toxic metabolites in their system.

Figure 9. Reproduction of bacteria

Figure 10. Bacterial Growth Curve a lag phase b log phase c stationary phase d lethal phase
Biochemical activity
- The most important biochemical and enzymatic systems of bacteria in milk and dairy products responsible for the following effects:
- carbohydrate digestion,
- protein digestion,
- fat digestion,
- digestion of lecithin,
- production of pigmentation,
- production of mucus (stringiness),
- production of odor,
- oxygen reduction,
- diseases.
Carbohydrates degradation
The breakdown of carbohydrates takes place:
- hydrolysis,
- alcoholic fermentation,
- lactic acid fermentation,
- Coliform-type of fermentation,
- butyric acid fermentation.

Figure 11. Lactose and sucrose degradation
Proteins degradation
Proteins are broken down into peptides and amino acids, caused by protease and peptidase enzymes.

Figure 12. Breakdown of proteins to amino acids by proteinase and peptidase enzymes
Fats degradation
A process is called lipolysis and the enzymes that carry out this reaction are lipases. Fats are esters of glycerol and fatty acids, so with lipase free fatty acids and glycerol are created.

Figure 13. Breakdown of lipids into free fatty acids and alcohol glycerol

Figure 14. Lipolysis – due to damage to the globular membrane the lactic fats release fatty acids.
Degradation of lecithin – lecithin is a phospholipid found in the membranes of fat globules and thus stabilizes the emulsion of fat and milk in cream. Lecithinase enzymes break down lecithin and thus destabilize the emulsion, so the fat is released from the globules and rises to the surface of milk or cream as greasy blots or curds.
Pigments and colors – bacteria that can create colors are called chromogenic bacteria. The color of their colonies is in the name of bacteria. An example is Staphylococcus aureus, whose colonies are golden yellow on a nutritious substrate.
Mucus production – some bacteria produce mucus consisting of polysaccharides that increase milk viscosity because they are soluble in milk. Such bacteria are also used to produce some fermented products, such as Scandinavian sour milks; e.g. Långfil from Sweden.
Production of odor – some bacteria produce scents that are characteristic of that species and that is the fresh aromatic smell of fermented dairy products that is provided by selected cultures of lactic acid bacteria.
Pathogenic microorganisms in raw milk
Some microorganisms can cause food poisoning (pathogenic microorganisms), either through intoxication and/or infection. Intoxication means the production of poisons (toxins) in food before the consumption of such foods. Infection means the entry, settlement, active growth and reproduction of such microorganisms in the human body. It often takes a large number of pathogens to cause infection. Sometimes, as in the case with Salmonella typhimurium minimum infectious dose (MID) can be just one bacterium. Pathogenic bacteria cause diseases in humans, animals and plants.
Pathogens in milk
-
Infectious
- Mycobacterium bovis
- Mycobacterium tuberculosis
- Escherichia coli (certain strains)
- Listerria monocitogenes
- Salmonella
- Campylobacter
- Corynebacterium diphteriae
-
Toxin makers
- Bacillus cereus
- Clostridium perfringens
- Staphyloccus aureus (certain strains)
Bacteria in milk
From a cow – milk is actually sterile when excreted from the udder. However, before it comes out of the udder, milk is contaminated with bacteria that penetrate from the outside into the teat canal and these bacteria are harmless and few in numbers under normal circumstances (up to several hundred per milliliter). In case of bacterial inflammation of the udder (mastitis), milk can be contaminated with a large number of bacteria, including pathogens, and thus becomes unfit for use. Such a state of udder also causes great suffering to cows. In the case of mastitis, the concentration of bacteria in the canal is high, so a large number of them is washed off at the beginning of milking in the first stream and for this reason the first stream from each teat is collected into separate vessel with a black background.

Figure 15. Bacterial entry through the teat canal and causing infection.
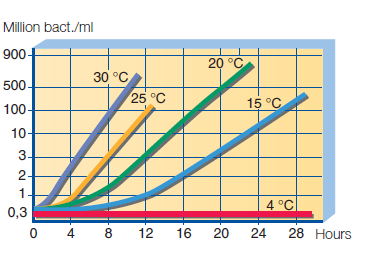
Figure 16. Collecting the first milk stream from each teat into a separate container with a black background.
In milk from cows suffering from mastitis white snowflakes and lumps are immediately seen as a sign of inflammation of the udder on a black background. Today, there are new methods and tests for mastitis as well as new instruments for detecting mastitis based on measurements of electrical conductivity, i.e. milk resistance. New methods and procedures are being introduced to determine the number of somatic cells in milk that are an indication of health status of udder. Somatic cells are epithelial cells of the udder and blood cells (granulocytes, lymphocytes, and leukocytes). An increased number of somatic cells indicates inflammation of the udder (mastitis). Healthy cow milk mainly contains less than 200 000, but also less than 100 000 somatic cells/ml. Raw milk for thermal processing may have a maximum of 400 000 somatic cells/ml. Filtering milk with the aim of removing somatic cells is an important procedure because it also removes bacteria accumulated on epithelial cells.
Contamination on the farm – on the farm, depending of the manipulation with milk, it can be contaminated with various microorganisms, but mostly with bacteria. The degree of contamination and the content of the bacterial culture depends of the cleanliness of the environment and the purity of the surfaces with which milk comes into contact. Most often it can be a milking machine or its components; vacuum assembly, milk bucket, milk filter/strainer, transport bucket, milk chiller, blender, etc. Surfaces that come into contact with milk are usually greater sources of contamination than just the cow’s udder. If cows are milked manually, bacteria can enter the milk from milker, cow, litter and air in the barn. The magnitude of the influence of each factor depends on the education and knowledge of the milker about the hygienic principles and the way in which the cow is treated. Many of these causes of milk contamination have been eliminated by the introduction of milking machine. Here caution is also required because a large number of bacteria can get into milk if milking equipment is not cleaned, disinfected and maintained properly as recommended by the professionals.
Bacteria in raw milk – milk is very nutritious and susceptible to pollution and growth of a wide range of bacteria. If it comes from a farm where hygiene is practiced, milk contains several thousand bacteria per ml. If on the farm cleaning, washing, disinfection and cooling of milk is not exercised correctly, the number of bacteria is measured in millions. Therefore, daily procedures in maintaining hygienic conditions on the farm, milking and cooling space are a determining factor for the bacteriological quality of milk. The maximum permissible number of live bacterial cells in raw milk can be 100,000 CFU/ml. Colony-forming unit (CFU) means the number of bacterial entities that have created visible colonies on a nutritional substrate. Under optimal conditions, a number of bacteria of less than 20 000 CFU/ml can be achieved, and this is an approximate number of living bacteria. The milk temperature when leaving the udder is about 37 °C. Rapid cooling of milk to a temperature between 4 °C and 2 °C greatly contributes to the quality of milk on the farm. Also, the treatment significantly slows down the bacterial growth in milk and preserves quality. The effect of temperature on the bacterial growth in raw milk is shown on the graph, Figure 17.
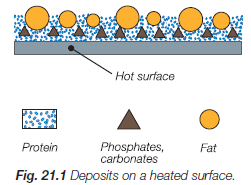
Figure 17. Effect of temperature on bacterial growth in raw milk.
Table 1. Presence of main bacterial groups in low-CFU milk
| Group | Frequency (%) |
| Micrococcus | 30 – 99 |
| Asporogenic (+)Gram; sticks | < 10 |
| (-)Gram; sticks | < 10 |
| Sporogenic | < 10 |
| other | < 10 |
Cleaning of dairy equipment
Aspects of cleaning – cleaning of dairy equipment stems from three important aspects of the operations of dairy companies:
- commercial obligations,
- moral obligations,
- legal obligations.
Cleaning goals – when we talk about cleaning results, the degree of purity is defined by the following terms:
- physical purity – removing visible impurities from all surfaces,
- chemical purity – removal not only of visible impurities, but of microscopic residues that can be determined by smell or taste, but not by a bare eye,
- bacterial purity – achieved by disinfection,
- sterile cleanliness – the destruction of all microorganisms.
It is necessary to know that the equipment can be bacteriologically clean without being physically or chemically clean. The surface of the heat processing device may remain covered with deposits of milk that have not been removed with washing. Such a surface, i.e. device can be sterilized, but it should be noted that the milk deposit is actually an isolation layer, so the milk temperatures during heat treatment will be lower than required. Such a situation should not be avoided because it brings uncertainty into the very effect of heat treatment of milk. Consequently, the equipment is washed to a pure metal glow of the work surfaces. Nevertheless, it is easier to achieve bacterial purity if the treated surface at least is firstly made physically clean. In cleaning procedures in dairies, the cleaning goals are almost always the same, and that is to achieve chemical and bacteriological cleanliness. For this reason, the surfaces of the equipment are first washed well with chemical detergents, and then disinfected.
Impurities on the surfaces of dairy equipment – milk ingredients get stuck and deposited in layers, and in/around these layers bacteria use that same impurities as their hiding place.

Figure 18. Deposits on heated surface
Heated surfaces – if the milk is heated to temperatures above 60 °C, milk deposits begins to form. Milk deposit consists of calcium and magnesium phosphates, carbonates, proteins and fats. These deposits are easy to spot on the heat exchanger plates in the sections for milk heating and heat recuperation. Deposits are firmly stuck on the surface of the plates and their color is from whiteish to burnt brownish.
Cold surfaces – a thin film of milk remains in pipelines, pumps, tanks, valves, etc. When the system is drained, washing must begin as soon as possible, otherwise the milk film on the surfaces will dry out and it will be more difficult to remove.
Cleaning procedures – in the past cleaning and washing were done by people with brushes and solutions of detergent (this is done even today in some cases), this is a difficult and not always effective approach, because the product can be contaminated with incompletely cleaned surfaces of equipment. To achieve good washing and disinfection of all segments in the production facility, circulatory Cleaning–in-place (CIP) systems have been designed and developed to make this possible. In order to achieve the desired degree of cleanliness, operations must be carried out strictly in accordance with the prearranged rules and procedures. This means that each time all sequences of the procedure must be the same.
The cleaning cycle in the dairy covers the following stages:
- return of product residues by scraping, draining and dislodging them with water or compressed air,
- pre-washing with water to remove residual impurities,
- detergent washing,
- rinsing with clean water,
- disinfection with heat (hot water) or with a chemical agent (optional); if this step is included the cycle ends with the final rinse with good quality water.
Each stage requires a certain amount of time to achieve an acceptable result.
Return of product residues – all product residues from the equipment in production lines must be dispossessed and collected at the end of the production process. This applies to tanks, pipelines, valves and machines, e.g. the butter production machine.
This achieves the following:
- reduce product losses,
- facilitates cleaning,
- reduces the load on wastewater.
Before starting cleaning (washing), the remaining milk is purged from production lines with water.
Pre-washing with water – is carried out as soon as possible after the cessation of production, because over time the remnants of milk would dry and stick to the surfaces and thus make it difficult to wash. The remnants of milk fat are much easier to rinse if the pre-wash water is warm, but the temperature should not exceed 55 °C, to avoid protein coagulation. The pre-wash must continue until the water at the exit of the system becomes clear. Any remaining impurities increases the expenditure of detergents. Good pre-wash can remove 90% of softer (unburned) residues, or 99% of total residues.
Washing with a detergent – impurities on heated surfaces are normally washed with alkaline and acidic detergents, in this order. After lye, the washing facility is washed with water.
Cold surfaces are normally washed with lye and only occasionally with an acidic agent. The main substance in alkaline detergents is sodium base (NaOH). To make better contact between the NaOH solution and the dirt-film, substances are added to the detergent to reduce the surface tension of the water and thus improve the soaking. Detergents must also allow the dispersion of impurities and the closure of suspended particles into capsules, hence preventing flocculation. To ensure satisfactory results with a particular detergent solution, important variables must be carefully controlled:
- concentration of detergent solution,
- detergent solution temperature,
- mechanical effect on the treated surface (flow rate),
- duration of washing (time).
Detergent concentration – before start of washing the amount of detergent in the solution must be adjusted to the correct concentration, while during washing the solution is diluted with rinsing water and milk residues. Partial system neutralization may also occur. As a result, it is necessary to check the concentration of the detergent during washing. If the check is not done, this may seriously affect the result of washing. This can be checked manually or automatically. Dosing must always be according to the supplier’s instructions, as increasing concentration does not have to improve the washing effect as due to increased foaming it can also have the reverse consequence. Excessive use of detergents makes washing unnecessarily more expensive.
Detergent temperature – the efficiency of the detergent solution is generally increased by increasing the temperature. Mixed detergents always have the optimum temperature, which must be applied in the washing process. Practice has shown that washing with an alkaline detergent must be carried out at the same temperature to which the product was exposed, but not less than 70 °C. Temperatures of 68-70 °C are recommended for washing with acidic detergents.
Mechanical washing effect – with manual cleaning brushes are used to achieve scraping of impurities from surfaces. In the case of mechanized washing of pipelines, tanks and other process equipment, the mechanical effect is achieved by the flow rate of the detergent solution. The detergent pressure pumps are dimensioned for higher capacities than the product pumps, as they need to retain velocity of 1.5-3 m/s in the pipes. At these flow rates, the current is very turbulent and such flow mechanism results in a good scraping effect on the surfaces of the equipment.
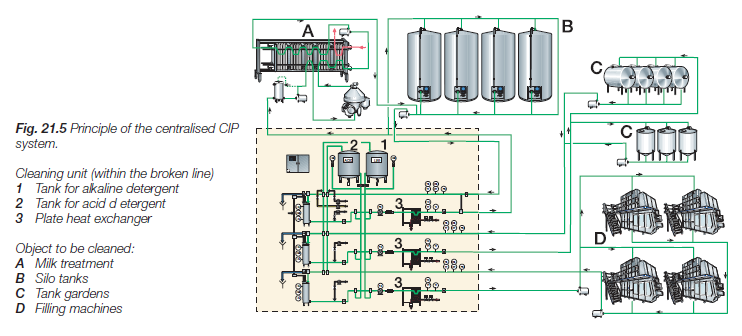
Figure 19. Mechanical cleaning effect
Washing time – work with detergent must be careful to achieve optimal effect. At the same time, the costs of electricity, heating, water and operation must be considered. On the other hand, it is not enough just to release the detergent solution through the pipeline system, rather the detergent must sufficiently circulate for the impurity to dissolve. The time it takes to achieve this depends on the thickness of the deposit and the temperature of the detergent solution. Plates of heat exchangers with hardened coagulated proteins must be treated with nitric acid in circulation for about 20 minutes. To dissolve the milk film from the walls of the tanks it is enough to treat it with an alkaline agent for 10 minutes.
Rinsing with clean water – after washing with a detergent, the surfaces must be rinsed with water long enough to remove all traces of the detergent. Remaining detergent residues in the system after washing can contaminate milk. All parts of the system must be completely drained after rinsing, and softened water should be used for rinsing. This prevents the formation of limestone on washed surfaces. Hard water with a high calcium salt content should be softened on ion exchangers at 2-4 °dH (German hardness degrees). After such a procedure the equipment and pipelines are practically sterile.
Disinfection – the effect of bacteriological cleaning can be improved only by disinfection of the entire system. Dairy equipment can be disinfected in two ways:
- thermal disinfection (boiling water, hot water, steam),
- chemical disinfection (iodophores, hydrogen peroxide, peroxi acetic acid etc.).
Disinfection can be carried out in the morning, just before starting milk processing. Milk can be received as soon as the disinfectant is completely removed from the system.
Cleaning-in-place (CIP) – systems are an integral part of smaller, medium and large production systems, Figure 20. Rinsing water, detergent solutions and hot water for disinfection circulate through tanks, pipes and process lines without dismantling the equipment, in closed washing circles. CIP technology is a special professional field that includes a wide range of scientific and technical disciplines. Hygiene in the process of milk production and processing must be seen in entirety as a very important factor in achieving the final goal, which is a quality dairy product. A prerequisite for a quality dairy product is above all quality milk.

Figure 20. Operational pronciples of the central CIP system
A good dairy product must have:
- good appearance,
- attractive aroma,
- good taste,
- nutritional value,
- health safety,
- expiry period.
Achieving these objectives requires an interdisciplinary approach covering agronomy, veterinary medicine, milk processing technology and the chemistry of washing and disinfection agents. All these disciplines also have their own important construction, mechanical and supervisory control components. Below we will analyze all possible causes of milk processing contaminations from barn to finalized product, with a focus on microbiological and chemical contamination.
The most important quality factors of produced milk
Milk production begins with the feeding of dairy cows, and ends with cleaning and disinfection of milking and cooling equipment. For this reason, the milk producer must master the following processes:
- production of feed,
- feeding
- milking
- squeezing
- cooling milk,
- cleaning and disinfection of milking and cooling equipment.
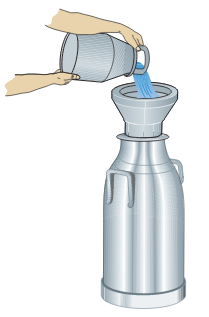
Figure 21. Preparation of a cow for milking involving cleaning and massage of udder.
In doing so, one must know the influence of breeding technology in its entirety and care for its own hygiene.
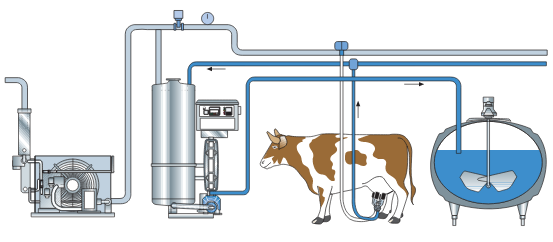
Figure 22. Milk flow in the rapid cooling system from the cow and all the way to the cooling tank.
One must also know the possible causes of milk contamination and how to reduce their effects which means:
- control of health status in dairy cows, especially udder,
- disposal of the initial milk streams,
- cleaning and disinfection of the teats before milking and their disinfection immediately after milking,
- proper milking routine,
- milk straining and rapid cooling, Figure 23.
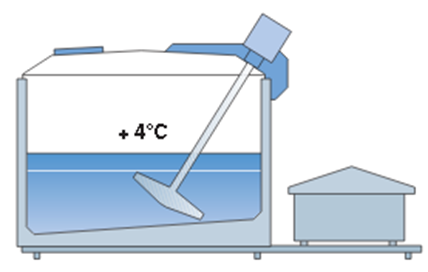
Figure 23. Refrigeration tank with mixer and cooling unit
In addition, one has to take care of:
- personal hygiene,
- hygiene in the barn,
- cleanliness of milking equipment.
Unclean milking equipment is potentially the strongest and most dangerous source of milk contamination with microorganisms, so the cleanliness of the equipment is the most important condition for preventing microbial contamination. Where and to what extent do microorganisms come to milk? The sources of microorganisms in milk are shown in Table 2.
Table 2. Sources of microorganisms in milk
| Source | Number per ml of gathered milk |
| air from the barn | up to 15000 |
| hands | up to several thousands |
| teat’s surface | up to 20000 |
| teat canal | up to 1000 |
| disease (incl. pathogens) | up to 20000 |
| milking equipment | from several thousand up to several millions |
From the presented data in Table 2 it can be concluded that all other efforts are futile if cleaning and disinfection of milking equipment is neglected.
The most common sources of contamination of raw milk – with manual milking, milk is mostly contaminated from barn air, milking vessels and the hands of milkers. Milking with damp hands is also inappropriate. With automatic milking by mobile device, all rubber parts of milking equipment are dangerous sources of contamination. In the case of a milking system (in a barn or at milking place) except the milking machine itself, milk taps, milk couplings, milk piping and connection points on the milk collection vessel are vital to care for. Attention must also be paid to equipment for the reception, cooling and storage of milk; milking vessels, milk chillers, i.e. plate cooler and cooled milk cisterns. After each milking, i.e. the depositing of milk, the equipment must be properly cleaned and disinfected.
The effect of cleaning is dependent of numerous factors:
- condition of the equipment (especially rubber parts that must be smooth and elastic),
- thorough removal of impurities from external surfaces of equipment (milking systems),
- rinsing the milking system with lukewarm water immediately after milking,
- the quality and concentrations of cleaning and disinfection agents used,
- the solution temperature,
- the duration of cleaning and disinfection,
- mechanical cleaning effect,
- rinsing with clean, drinking water to flush the residues of chemical agents.
Impurities in the production and processing of milk – the production of milk and dairy products with high bacteriological safety requires thorough cleaning and disinfection of all surfaces of dairy equipment and processing complex. Dairy equipment is the main source of contamination of the finished product, so the risk increases with the increased surface area of the equipment. Cleaning of equipment after the production is necessary because impurities and milk deposits must be disposed as they represent an excellent medium for the microbial growth that would contaminate the next batch of manufacturing. After cleaning, all surfaces must be disinfected so that all remained microbes and enzymes after cleaning are destroyed. Disinfection of poorly cleaned equipment is less effective because impurities and milk residues preserve and protect microbes from disinfectants. Besides that, remained impurities can inactivate disinfectants. Impurities in dairies consist of fats, proteins and mineral substances. Microbes bind to the layers of impurities and rapidly multiply under favorable conditions hence, for successful cleaning of dairy plants it is necessary to consider several important factors:
- good design and use of materials that are cleaned well and easy from remains of fresh milk, dried milk or burnt milk. Burnt milk contains denatured proteins, fat emulsions and calcium phosphate that make cleaning difficult,
- water in the cleaning process must be soft because mineral salts with milk components produce milk deposits,
- water must be fully bacteriologically safe,
- cleaning solution must have an optimum temperature (not below 33 °C at which milk fat is melted),
- in the pipes cleaning solution must flow turbulently to achieve effects of mechanical washing,
- the composition of the cleaning solution is especially important.
The process of cleaning and disinfection – cleaning and disinfection are two related procedures of the sanitation process, and they are performed as separate procedures. The effect of cleaning can be described as a product of chemistry, mechanics, temperature and weather. The remaining microbes are destroyed by disinfection, which follows after the cleaning. For this reason, pre-cleaned and washed surfaces should be disinfected.
Cleaning and disinfection are carried out in the following steps:
- physical, mechanical cleaning; removal of all visible impurities,
- dry cleaning; removal of visible and invisible defects for bare eye,
- rinsing of cleaning agents,
- disinfection
- last rinse (unless the disinfectant is peroxyoacence acid).
Cleaning agents
Water – detergents in the dairy industry are aqueous solutions of bases and acids for which two factors are important; conductivity and pH value. Conductivity is the ability of a substance or solution to conduct an electric current while the pH value is a negative logarithm of the concentration of hydrogen ions in an aqueous solution. Clean water has a pH = 7, which means that the concentration of hydrogen ions is equal to the concentration of hydroxy ions. The pH scale values are unambiguous, absolute numeric values from 0 to 14. By adding acid, the pH of water is lowered, and with the addition of bases, the pH increases. Acids have a pH value of less than 7 and basis greater than 7. Conductivity and pH value play an important role in automatic managing of the cleaning process in dairies. Water hardness is the amount of calcium/magnesium sulfates/carbonates and hydrogencarbonate dissolved in water. Water hardness is expressed in German degrees of hardness and has certain significance in cleaning processes. Water hardness is important in cleaning and rinsing processes because dissolved salts are deposited as water calculus in the production system. For this reason, partially softened water should be used for cleaning.
The composition of cleaning agents – given the range of pH value, cleaning agents are divided into alkaline, acidic and neutral. Alkaline agents contain bases, sequestrants, and complexons, tensides, anti-foaming agents, oxidative enhancers and solubilizers. Acidic agents contain acids, corrosion inhibitors, tensides (surface active substances) and anti-foaming agents.
Bases – sodium hydroxide is the most widely used base (NaOH). Due to the high pH value, it reacts with different components within impurities so that other compounds can easily emulsify them. Sodium carbonate is used with slightly alkaline agents (Na2CO3).
Acids – commonly used is nitric acid (HNO3) and phosphoric acid (H3PO4). Complexions, sequestrants, tensides and other components improve washing effects and prevent any negative consequences of using detergents. The concentration of active substances in the cleaning agents should be checked by alkalimetry and acidimetry with appropriate indicators.
Disinfectants – for use in the dairy industry must meet an entire range of requirements:
- a wide range of actions,
- rapid action at low temperatures,
- low toxicity,
- good rinsing,
- environmental friendliness,
- non-corrosivity,
- remnants of an agent must not harm the product,
- ability for automatic concentration control,
- good stability of concentrates and working solutions.
Hydrogen peroxide – Commercial agents contain 35, 50 and 70% H2O2. Hydrogen peroxide must be well stabilized, very clean and of high quality. Hydrogen peroxide decomposes to water and nascent active oxygen. It acts oxidatively on biologically active cellular systems and destroys them irreversibly, causing cells to die off. It is used at temperatures greater than 50 °C and is environmentally friendly.
Peroxyacetic acid (PAA) – is a disinfectant that acts oxidatively with high oxidation potential. Stabilized formulation is with 15% PAA in the equilibrium with hydrogen peroxide, water and acetic acid. PAA reacts with proteins in the wall of cellular membrane. It enters the cell like weak acid and destroys enzymatic systems and nucleic acids. It acts on all kinds of microorganisms, bacterial spores and viruses. It decomposes into active oxygen, water and acetic acid, so it must be rinsed from production systems.
Quaternary ammonia compounds – are surfactant active substances (tensides) that are adsorbed on the surface of microorganisms and by lowering the surface tension of water they affect the permeability of the membrane. This changes the surface structure of the cell, cancels the functions of the cell membrane and the cells die off. Due to the different structure of the cell wall, they have a weaker effect on the gram-negative bacteria.
Aldehydes – are compounds that react with amino groups of amino acids. Irreversible changes occur, causing damages to the cell wall and death of the cell. These disinfectants are most commonly used in dairy farming. For small plants, new environmentally friendly detergents and disinfectants are available on the market.
Cleaning methods – two cleaning methods:
- CIP procedure (cleaning-in-place)
- COP procedure (cleaning-out-place)
CIP process – is semi-automatic or fully automatic internal cleaning of production systems without disassembly.
Distinguishing:
- simple closed systems (circulation),
- smaller CIP systems for “lost cleaning,”
- fully automatic CIP devices with refluxed cleaning solutions, and the process is controlled by conductivity and pH values.
The advantages of CIP are: higher cleaning quality, increased safety, cost control.
COP procedure – is the external cleaning of equipment and production complex and cleaning of equipment from the inside, after the production lines are disassembled.
Cleaning is performed:
- manually
- with high-pressure appliances,
- with foam cleaners.
Despite the development of cleaning technique there is still a need for such cleaning.
Sampling and cleanliness control
The main purpose of hygiene in the plant is to ensure that the equipment does not contaminate the product. In the event of contamination, control must determine where bacteriological contamination, chemical contamination or contamination by some impurity has occurred. The method of controlling the effectiveness of cleaning and disinfection where sampling is easily performed is divided into three groups:
- control of all surfaces that must be cleaned after the new process
- control of all surfaces that must be cleaned only before the start of the new process (jars, cheese molds, etc…),
- indirect control; it is the control of solutions and methods that we use when cleaning; control of raw materials, semi-finished products and finished products.

Figure 24. Sample analysis – purity control
Control and sampling methods for determining the effectiveness of cleaning and disinfection in dairy plants include:
- visual control,
- sampling from the surfaces of dairy equipment and production complex,
- air sampling,
- sampling of water,
- sampling of raw materials and products.
Regular control of the health status and hygiene of staff, environmental hygiene, production complex in addition to hygienic quality of additives is important. The sampling equipment must be fully adapted to the function.
Basic guidelines for cleaning control – are based on the results of microbiological tests. Although these tests are the most important, still visual inspections, smells (chemicals) and physical analyses and proper data processing and their interpretation are also important. Sampling for microbiological tests must be carried out by trained staff. The frequency of sampling depends on the type of production, the quality of the equipment, the stability of the production process and finally on the company’s quality policy. Sampling must be accompanied by precise records of all relevant sampling circumstances. Contact surfaces are all surfaces that come into contact with milk. Particular attention must be paid to places that are difficult to clean; dents, joints, valves, probes etc. Controls must be done after cleaning and disinfection of the dairy equipment, i.e. of the process line before the start of production, to check that there has been zero recontamination. Direct methods of controlling microbial pollution are swabbing and rinsing. There is also a contact method by which a solid nutrient base is pressed against the tested surface. A new approach to these problems is the detection of ATP and AMP, as indicators for the presence of microorganisms.
Air sampling – air microflora plays a certain role in the contamination of the product when production for various reasons cannot take place in enclosed system. In this case, the air must be free from microorganisms, and keep the entire workspace under elevated pressure of practically sterile air. In such a space, the number of live microbes in the air must be controlled.
Control of cleaning solutions – before use all cleaning solutions also must be controlled for the concentration of detergent i.e. active substance.
Control of raw materials and finalized product – this gives a reflection of the hygienic status of the production process in the plant. The process can be divided into segments and thus locate the source of contamination. Milk producers keep their raw milk in properly and rapidly cooled containers or in a larger insulated container. The mobile cistern that collects milk in the field has a flexible hose and self-suction pump for gathering milk from the farmers that is transported to the reception ramp in the dairy. Critical points for possible contamination of milk are:
- the inner surface of rapid chiller, i.e. containers,
- valves and couplings,
- milk pumps,
- flexible hose.
After arriving at the dairy, raw milk is pumped into a container (tank) via filters, milk volume gauges and plate coolers for refrigerated storage. Critical points for possible contamination are:
- milk filter,
- quantity meter,
- plate cooler,
- raw milk container.
Raw milk from the milk container goes to the milk pasteurization department. This is the start of the milk processing.


